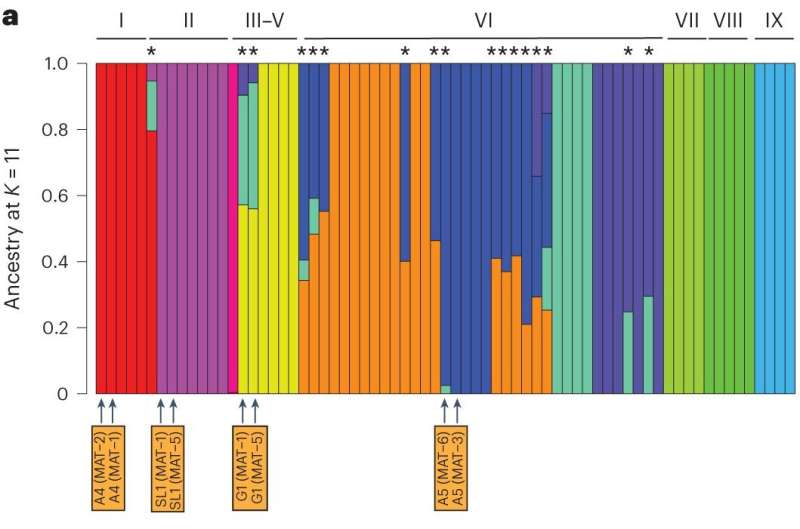
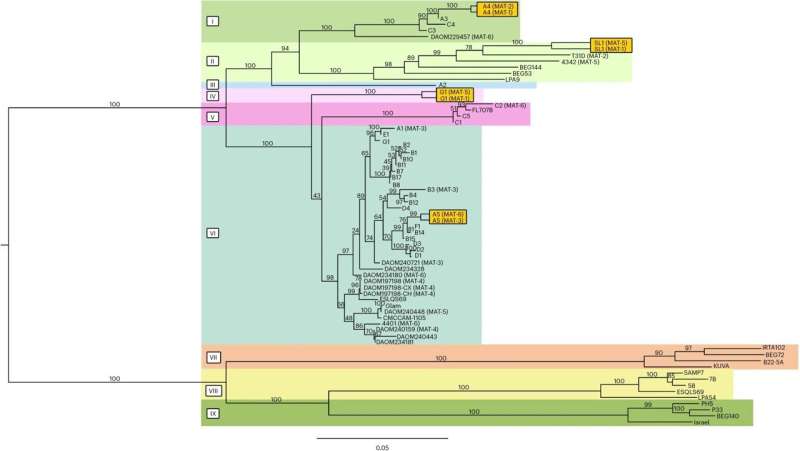
Saturday, 23 March 2024 08:16:28
PestNet
Grahame Jackson posted a new submission ‘A single laccase acts as a key component of environmental sensing in a broad host range fungal pathogen’
Submission
A single laccase acts as a key component of environmental sensing in a broad host range fungal pathogen
Nature
- Nathaniel M. Westrick,
- Eddie G. Dominguez,
- Madeline Bondy,
- Christina M. Hull,
- Damon L. Smith &
- Mehdi Kabbage
Communications Biology volume 7, Article number: 348 (2024)
Abstract
Secreted laccases are important enzymes on a broad ecological scale for their role in mediating plant-microbe interactions, but within ascomycete fungi these enzymes have been primarily associated with melanin biosynthesis. In this study, a putatively secreted laccase, Sslac2, was characterized from the broad-host-range plant pathogen Sclerotinia sclerotiorum, which is largely unpigmented and is not dependent on melanogenesis for plant infection. Gene knockouts of Sslac2 demonstrate wide ranging developmental phenotypes and are functionally non-pathogenic. These mutants also displayed indiscriminate growth behaviors and enhanced biomass formation, seemingly as a result of their inability to respond to canonical environmental growth cues, a phenomenon further confirmed through chemical stress, physiological, and transcriptomic analyses. Transmission and scanning electron microscopy demonstrate apparent differences in extracellular matrix structure between WT and mutant strains that likely explain the inability of the mutants to respond to their environment. Targeting Sslac2 using host-induced gene silencing significantly improved resistance to S. sclerotiorum, suggesting that fungal laccases could be a valuable target of disease control. Collectively, we identified a laccase critical to the development and virulence of the broad-host-range pathogen S. sclerotiorum and propose a potentially novel role for fungal laccases in modulating environmental sensing.
Read on: https://www.nature.com/articles/s42003-024-06034-7
Saturday, 23 March 2024 08:16:28
Grahame
Jackson posted a new submission ‘A single laccase acts as a key component of
environmental sensing in a broad host range fungal pathogen’
Submission
A single laccase acts as a key component of environmental sensing in a
broad host range fungal pathogen
Nature
- Nathaniel M. Westrick,
- Eddie G. Dominguez,
- Madeline Bondy,
- Christina M. Hull,
- Damon L. Smith &
- Mehdi Kabbage
Communications
Biology volume 7,
Article number: 348 (2024)
Abstract
Secreted laccases are important enzymes on a broad ecological scale for
their role in mediating plant-microbe interactions, but within ascomycete fungi
these enzymes have been primarily associated with melanin biosynthesis. In this
study, a putatively secreted laccase, Sslac2, was characterized
from the broad-host-range plant pathogen Sclerotinia sclerotiorum,
which is largely unpigmented and is not dependent on melanogenesis for plant
infection. Gene knockouts of Sslac2 demonstrate wide ranging
developmental phenotypes and are functionally non-pathogenic. These mutants
also displayed indiscriminate growth behaviors and enhanced biomass formation,
seemingly as a result of their inability to respond to canonical environmental
growth cues, a phenomenon further confirmed through chemical stress,
physiological, and transcriptomic analyses. Transmission and scanning electron
microscopy demonstrate apparent differences in extracellular matrix structure
between WT and mutant strains that likely explain the inability of the mutants
to respond to their environment. Targeting Sslac2 using
host-induced gene silencing significantly improved resistance to S.
sclerotiorum, suggesting that fungal laccases could be a valuable target of
disease control. Collectively, we identified a laccase critical to the
development and virulence of the broad-host-range pathogen S.
sclerotiorum and propose a potentially novel role for fungal laccases
in modulating environmental sensing.
Read on: https://www.nature.com/articles/s42003-024-06034-7